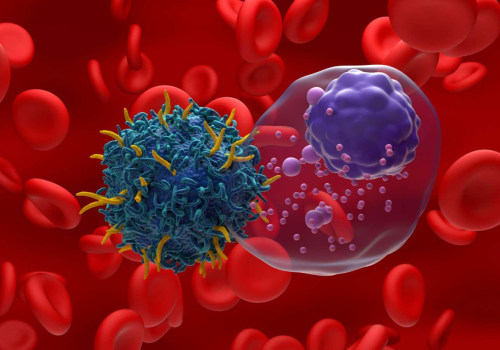
With this treatment, a specialist nurse collects T cells and then they are sent to the laboratory, where a change is made to the T cells to become CAR T cells. After a few weeks, a droplet containing these cells returns to the bloodstream. CAR T cells then recognize and attack the cancer cells. CAR T cell therapy involves the genetic engineering of the patient's own T cells (red) to attack cancer cells (red and blue). CAR T-cell therapy is a new type of cancer treatment that uses the immune system to kill cancer cells.
In some cases, it has cured people when all other treatments have failed. CAR T-cell therapy is a personalized form of immunotherapy that trains the immune cells themselves to recognize and destroy cancer. It can be a powerful option for treating certain types of blood cancer that are difficult to treat, especially when other treatments are no longer effective. Chimeric antigen receptor (CAR) T-cell therapy treats some types of blood cancer.
Scientists create the treatment by adding a laboratory-made gene to cancer-fighting T cells. This change helps T cells detect and kill cancer cells. Health care providers can use this treatment when other treatments aren't effective or when blood cancer comes back. The European Society for Blood and Bone Marrow Transplant (EBMT) collects information on the different types of transplants and cell therapies performed in Europe, including CAR T cell therapies.
Researchers at the University of Colorado's Anschutz medical campus have successfully developed a new version of CAR-T cell therapy that can improve cell efficacy and longevity, especially against cancer cells, which are more difficult to detect and combat in previous CAR-T treatments. On the other hand, an analysis of research on CAR T cell therapy concluded that, for a significant percentage of people, CAR T cell therapy it wasn't a cure for his condition. Accordingly, other approaches described in this review that are currently being tested show great potential, such as an autologous T-cell therapy with TCR called afami-cel for synovial sarcoma129 and other cell therapies for HPV-associated cancers. This review provides an overview of the current development of CAR-T cell therapies for hematological and solid tumors, while examining the challenges associated with their application in AML, ongoing clinical trials, and future directions for optimizing CAR-T cell therapy in the treatment of AML.
This review describes that, while no CAR-T treatment has received FDA approval for solid malignancies, certain strategies, such as intracranial CAR therapy aimed at IL-13RA (multifocal glioblastoma) 12 and ongoing clinical trials with claudin18,2 (gastrointestinal tumors12) and GD2 (glioma mutated in H3K27m-125) and neuroblastoma12, are promising.